It appears to be an achievement to launch the first thousand bottles of a beverage. Yet, the real challenge begins when brands move from beverage pilot to commercial production. This shift is known as the scale-up shock and exposes hidden complications. The flavour changes, it becomes less carbonated, there is a decrease in the shelf life and the price increases.
In this blog, we’ll explore the realities of commercial beverage production, the beverage scale-up process, and the common beverage scale-up issues that delay startups if SOPs are incomplete.
Scaling beyond 1,000 bottles? Avoid hidden production risks with SOP-driven manufacturing planning.
Challenges After Scaling Beverage Production
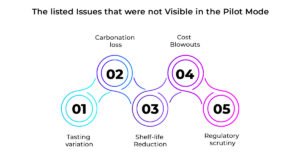
At a larger scale that requires more than 1,000 bottles, issues that were not visible in the pilot mode emerge in the brands:
- Tasting variation: In the absence of controlled mixing and heating, tastes vary across samples.
- Carbonation loss: CO₂ retention differs at volume, leading to flat beverages.
- Shelf-life reduction: Stress from larger batches exposes microbial and stability weaknesses.
- Cost blowouts: Wastage ignored at pilot scale becomes financially damaging.
- Regulatory scrutiny: Informal QC practices fail under compliance checks.
These beverage scale-up issues highlight why process discipline matters more than recipe creativity.
Pilot vs. Commercial Beverage Production
| Area | Pilot (≤1,000 bottles) | Commercial Scale (10,000+) |
|---|---|---|
| Mixing | Manual judgment | Time & RPM controlled |
| Carbonation | Adjusted on feel | Pressure & temperature SOP |
| Flavour | “Okay” taste | Identical batch-to-batch |
| Shelf Life | Estimated | Validated & documented |
| QC | Informal | Defined acceptance limits |
| Costing | Wastage ignored | Every % matters |
This table shows why commercial beverage production requires SOP-driven precision. Without it, brands scale chaos, not quality.
Recommended Read – Commercial Beverage Formulation
Why SOPs Are Critical in Beverage Scale Up
Incomplete SOPs create bottlenecks and chaos. A structured framework ensures:
- Recipe control: Ingredient order, temperature, and mixing time locked.
- Raw material assurance: Vendor specs, QC parameters, and traceability defined.
- Process validation: Heating, cooling, carbonation, and filtration standardised.
- Packaging reliability: Fill volume, headspace, sealing, and coding verified.
- Quality checkpoints: Brix, pH, sensory, and microbiological testing documented.
- Shelf-life stability: Stress testing and validation protocols in place.
Startups that ignore SOPs often face rejections, distributor complaints, and relaunch costs.
Case Study: From Pilot Success to Commercial Failure
Being a drink startup based in India, the company had introduced a carbonated hibiscus drink, which had been sold at 800 bottles through cafes and online platforms in 6 weeks. The positive customer response led to customer feedback and repeat orders, which motivated the founders to go commercial, that is, to 12,000 bottles on a contract manufacturing plant.
What went wrong at scale:
- The carbonation decreased by around 35 % in the space of ten days after bottling, making the drink flatten in the retail shelves.
- Darkening of colour occurred due to uncontrolled heating during the preparation of the syrup.
- The mixing time and the RPM were not standardised, which led to flavour drifting between the early and late batches.
- 18% of the bottles did not pass through distributor quality control as a result of the uneven volume of the fill and low CO₂.
Root causes identified during investigation:
- There was no standard-operating procedure that was established to combine the speed, time, or temperature restrictions.
- Chilling temperature and pressure of carbonation were at the discretion of the operator.
- No Brix or pH monitoring of the in-process was noted.
- The CIP cycles differed with batches and posed increased microbial risk.
Outcome:
The brand withdrew 3,200 bottles, two regional distributors, and it took the company almost six months to re-engineer its process and documentation and then re-introduced the brand.
Insight:
The case shows that the production of commercial beverages does not fail due to inexpensive recipes; rather, it is not a discipline of SOP. This case is not an example of one brand’s failures, but multiple to learn from.
Foodsure SOP Framework for Scale-Up Stability
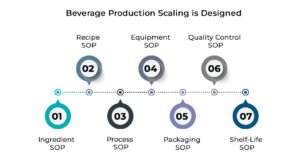
At Foodsure, beverage production scaling is designed to be done in a layered SOP system in which we create consistency before volume is added. This model will ensure that a certain process, which worked well with 1,000 bottles, will also work the same way with 100,000 bottles.
-
Ingredient SOP
- Vendor list, raw material specification Authorization.
- Parameters of incoming quality control (Quality Check (QC)).
- All of the traceability is on a lot level.
-
Recipe SOP
- The sequencing order of the addition of ingredients.
- Limitations of the temperatures of each stage.
- Confusion of time standards and RPM standards.
-
Process SOP
- Heating and cooling curves
- Pressure and temperature settings for carbonation.
- Time limitations for filtration and holding.
-
Equipment SOP
- Machine parameter locks
- CIP and sanitation cycles
- Premature maintenance programmes.
-
Packaging SOP
- Fill volume tolerances
- Headspace control
- There are balance of balance and seal verifications.
-
Quality Control SOP
- CO2, pH and Brix receiver limits.
- Sensory benchmarks
- The frequency of microbiological testing.
-
Shelf-Life SOP
- Stability test procedures.
- Check the condition of storage.
- Expiry Logic and documentation.
Why this matters:
Using this SOP-based practice removes operator variation, batch defect or failures and maintains brand consistency at the rate.
Related Insights for Beverage Scale-Up
Conclusion
The leap from pilot to commercial production is where beverage brands are made, or broken. Commercial beverage production demands SOPs that transform recipes into repeatable processes. Those who invest early in documentation scale smoothly; those who don’t end up firefighting.
At Foodsure, beverages are engineered to scale under one controlled, SOP-driven shed. If you’re preparing for growth, ensure your SOPs are complete before scaling.
Ready for Commercial-Scale Beverage Production?
Foodsure helps brands transition from pilot batches to SOP-driven commercial production with consistent quality, validated shelf life, and manufacturing-ready systems.
Trusted by 100+ beverage founders and FMCG brands for scalable manufacturing solutions.
Frequently Asked Questions
What is scale-up shock in beverage production?
It’s the set of challenges brands face when moving from pilot batches to commercial scale.
Why does taste change after scaling?
Larger volumes alter heat transfer, mixing, and ingredient interaction.
How does carbonation differ at scale?
CO₂ retention depends on pressure and temperature, which must be SOP-controlled.
What are common beverage scale-up issues?
Taste drift, carbonation loss, shelf-life reduction, and cost blowouts.
Why are SOPs critical in commercial beverage production?
They ensure consistency, compliance, and traceability across batches.
What happens if SOPs are incomplete?
Brands face rejected regulatory penalties and consumer dissatisfaction.
How does shelf life change after scaling?
Larger batches expose microbial and stability weaknesses, requiring validated protocols.
What role does QC play in scale-up?
QC defines acceptance limits for parameters like Brix, pH, and sensory evaluation.
Can startups skip SOPs in early stages?
Skipping SOPs may work at pilot scale, but causes chaos at the commercial scale.
How can Foodsure help beverage brands scale?
Foodsure provides turnkey, SOP-driven solutions to ensure smooth scale-up and brand consistency.