The Freeze Drying (or Lyophilisation) procedure is a kind of very highly developed and proven technique in lyophilisation service providers in the Food, Pharmaceutical, Nutraceutical and Biotech Industries, It is an ideal solution for large corporations that sell food products worldwide, due to the ability to easily ship frozen, dehydrated products. Consumers are increasingly demanding fresh, superior quality products with a longer shelf life, making this an extremely valuable technology for manufacturers. There is a growing demand for Freeze Dried technology and Freeze Dried products to create Specialty Meals, Medications and Freeze Dried Fruits and Vegetables.
This blog will explore the complete freeze-drying process in detail, as well as the advantages of utilizing Freeze Drying technology in today’s market and how Freeze Drying technology is rapidly becoming the most prominent method for manufacturing freeze-dried goods around the world.
Want to optimise your Food Business? Request a free consultation!
Food Product Development Services – Click Here
Understanding Freeze Drying Technology& Formulation 
Lyophilisation is a process of dehydration that absorbs all water content from a product without using any damaging heat. This procedure is done in a series of steps, which are all regulated. At first, the product is frozen to very low temperatures to establish a stable structure. This is followed by a phase of drying in which the pressure is lowered, and the ice that is frozen water is converted directly to vapour in the form of gas. The last step is called secondary drying, which leaves the product dry and removes any residual moisture to make sure that it is stable over a longer period of time. The drying phase takes place whenever there is a reduction of pressure and the water ice is converted directly to vapour. The very last step of the process, secondary drying, removes residual moisture, which ensures the product is stable until the dry condition of the product is stable over the long run.
The freeze drying formulation used for freeze-drying is one of the most important factors in creating product specification, ensuring quality and reliability in commercial applications. It is especially critical for businesses providing B2B products in the food, nutraceutical, pharmaceutical, and biotechnology spaces. Unlike traditional commercial drying methods, the freeze-drying process will help preserve the original form, flavor, and bioactive properties of sensitive materials provided the formulation used is designed accordingly. When developing successful formulation for use in B2B applications, freeze-drying should be focused on selecting component carrier systems that are compatible, optimizing solid content, and managing moisture so that each individual piece within a batch can have a consistent and even drying rate and hydration rate. Formulations that are not optimized correctly could cause collapse, powder loss or decreased functional capabilities.
You May Also Like: Food Packaging and Labelling
How Freeze Drying Differs from Conventional Drying Methods
| Aspects | Traditional Drying Method | Freeze Drying |
| Drying method | Uses heat to evaporate moisture | Removes moisture through sublimation without heat |
| Effect on product texture | Texture is often altered or collapsed | Original structure preserved on a large scale |
| Flavour / aroma | Partial loss due to heat exposure | Natural flavour and aroma retained |
| Nutritional value | Heat-sensitive nutrients may degrade | High retention of nutrients and bioactives |
| Rehydration | Slow and incomplete | Fast and nearly complete rehydration |
| Shelf life | Moderate, often needs preservatives | Long shelf life without preservatives |
| Commercial focus | Speed and cost efficiency | Quality, stability, and premium value |
| Best suited for | Mass-produced, low-cost products | Higher value, sensitive products |
Key Benefits of Freeze-Dried Products 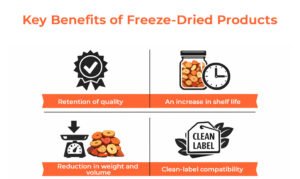
- Retention of quality: Freeze drying is all about minimising the temperature of heat damage while maintaining the natural colour, flavour, texture, and nutritional value of the food or the pharmaceutical product.
- An increase in shelf life: Because of ultra-low moisture levels, there are very few opportunities for microbial growth and enzyme activity; thus, when packaged appropriately, products can be kept stable for years, enabling access to global markets.
- Reduction in weight and volume: Since products absorb moisture, they can be made lighter and more compact, reducing costs for transport and inventory management, especially for the provision of emergency nutritional supplies to military personnel and for other purposes associated with defence and export supplies.
- Clean-label compatibility: No chemical preservatives are required in the process of freeze drying. Therefore, it is easy to produce clean-label or minimally processed products that are safe to consume and have a long shelf life without sacrificing either of those qualities.
Role of Freeze Drying in the Food Industry
The food sector is the biggest commercial user of freeze drying technology. There is an extensive range of freeze-dried food products, including fruits, vegetables, herbs, and spices, as well as instant coffee, dairy ingredients, ready-to-eat meals, and functional powders. Freeze drying enables food manufacturers to maintain the quality attributes of the foods while allowing rapid rehydration. This leads to superior taste, mouthfeel, and acceptability of the foods as compared to the traditionally dried foods.
Freeze drying also promotes the development of novel products containing heat-sensitive components, natural colours, and other functional ingredients. It is premium in snacking and health food sectors as well as in space-efficient nutrition products, and in formulations developed with a focus on exportation, where quality consistency is also paramount.
Pharmaceutical and Biotechnology Applications
Freeze Drying a critical process in the manufacture of drugs. Several active pharmaceutical ingredients API’s that need to be freeze-dried include vaccines, biologics and injectable formulations and are sensitive to both moisture and heat. By freeze-drying these materials, Pharmaceutical companies can ensure longer shelf life with room temperature storage capabilities. This will greatly facilitate the transportation of these products to many parts of the world where cold-chain access is limited. Freeze-dried Pharmaceuticals are much more portable, storable and formulatable; they retain all of their safety and efficacy. Freeze-drying is used by many biotechs to maintain the integrity of enzymes, cultures, probiotics, and diagnostic reagents. In view of stringent requirements on maintaining biological activity of these materials, many biotechs prefer to use freeze-drying over other methods of preservation of biological activity.
Industrial and Speciality Uses
Beyond food and pharmaceuticals, freeze-drying serves speciality industries where consistent performance is critical. For example, a cosmetics company uses freeze-drying for their botanical extracts and active ingredients’ preservation. A pet food brand has developed a premium high-protein raw-style product using freeze-drying, and researchers also utilise this method of preservation to keep samples and reagents in their original state. For these industries, concern about the costs associated with processing products through freeze-drying is secondary to their concerns about the reliability and functional integrity of the product produced.
Freeze Drying Equipment and Process Control
The sophisticated, highly automated nature of modern Freeze Drying Systems means that the Freeze Dryer, Vacuum System, Condenser, Heating Shelf, and PLC Automation are all integrated into one complete system. Freeze Drying Systems can be designed/configured in both Batch and Continuous designs, and are available in a wide variety of sizes, including small pilot-scale R&D Freeze Dryers, to large Industrial systems. The ability to design the equipment in this manner, and the ease with which companies can build a Product from Pilot scale and move to Commercial scale Production without having to redesign the entire process, are tremendous advantages.
Challenges and Operational Considerations
Among the various advantages of freeze drying, some disadvantages and challenges must be acknowledged. First, there are the high capital costs. Second, the process is extremely time-consuming, and the energy costs can be extremely high. These challenges can be enhanced with new system designs, energy-efficient methods, and optimisations that make use of the data that is collected.
Market Trends and Future Outlook
Global trends such as increased demand for nutraceutical and functional foods, growth of biologics and vaccines, consumer demand for clean-label products, and demand for products with longer shelf lives have all contributed positively to the growth of freeze drying. In particular, the demand for freeze-drying technology is growing in emerging markets, with the opportunity to produce high-value products and, in turn, provide better quality customer service.
Trending Right Now: High-Protein Snacks Development
Conclusion
Freeze drying represents the new positioning in innovation and flexibility. It serves as a high-quality preservation technique rather than simply a food preservation method. As such, freeze drying will preserve vitamins and minerals in their active state and have the capability to fulfil their intended functional purpose throughout their extended shelf life due to the high-quality preservation process. Economic viability of freeze drying will serve as a competitive advantage for those manufacturers heavily influenced by the quality assurance culture and environment.
Need Expert Help to Launch Your Food Product Legally & Successfully?
Foodsure supports food brands with FSSAI-compliant labelling, pre-launch checks, and regulatory compliance to ensure a smooth and legal food product launch. We help prevent penalties and delays before your product enters the market.
What We Help You Solve
-
FSSAI label review and correction
-
Nutritional panel accuracy and ingredient validation
-
Claim verification and compliance
-
Packaging artwork and readability checks
-
Audit, inspection, and documentation support
Who We Work With
-
Startups launching new food products
-
FMCG and D2C food brands
-
Private label and co-manufacturers
-
Importers, exporters, and suppliers
Frequently Asked Questions
What is freeze drying formulation?
Freeze drying formulation refers to designing ingredient compositions that remain stable, functional, and high quality during freeze drying and after rehydration.
Why is formulation important in freeze drying?
Proper formulation prevents structural collapse, poor rehydration, moisture retention, and loss of flavour or bioactive properties.
Which industries use freeze drying formulation the most?
Food, nutraceutical, pharmaceutical, biotech, and ingredient manufacturing industries widely use freeze drying formulations for sensitive products.
Can all ingredients be freeze dried?
Not all ingredients perform well without formulation adjustment. Some require carriers or stabilisers to maintain structure and stability.
How does formulation affect shelf life?
Optimised formulations reduce residual moisture, improve stability, and significantly extend shelf life without chemical preservatives.
What role do carriers play in freeze drying?
Carriers improve drying efficiency, prevent stickiness, and enhance powder flow and rehydration performance.
Is freeze drying formulation scalable?
Yes. When developed correctly at pilot stage, freeze drying formulations can be scaled reliably to commercial production.
How does formulation impact rehydration?
Good formulation ensures faster, uniform rehydration while retaining original texture, taste, and functionality.
Are freeze dried formulations regulatory compliant?
They can be fully compliant when ingredients, processing parameters, and documentation meet applicable food or pharmaceutical regulations.
When should formulation optimisation be done?
Formulation optimisation should be completed before commercial scale-up to avoid production failures and cost overruns.